ISO-9001-Lead-Auditor Exam Dumps - QMS ISO 9001:2015 Lead Auditor Exam
Searching for workable clues to ace the PECB ISO-9001-Lead-Auditor Exam? You’re on the right place! ExamCert has realistic, trusted and authentic exam prep tools to help you achieve your desired credential. ExamCert’s ISO-9001-Lead-Auditor PDF Study Guide, Testing Engine and Exam Dumps follow a reliable exam preparation strategy, providing you the most relevant and updated study material that is crafted in an easy to learn format of questions and answers. ExamCert’s study tools aim at simplifying all complex and confusing concepts of the exam and introduce you to the real exam scenario and practice it with the help of its testing engine and real exam dumps
During a second-party audit, the auditor examines the records that are available for the external provider, ABC Forgings, to whom manufacturing has recently been outsourced.
There are standard external provider checklists for three competitors for the contract and there are inspection records from the trial manufacturing batches produced by ABC Forgings. There is no documented evidence of the criteria used to confirm the appointment of ABC Forgings, and no contract or terms and conditions. Ongoing monitoring indicates that external provider performance is satisfactory, but no documented information has been retained.
Select two options for the evidence which demonstrates a nonconformity with clause 8.4 of ISO 9001.
You work as an external quality consultant for an organisation, 'A', which provides packaged food to the public. You are asked to lead a team (you as the leader and two other auditors) to audit a supplier, 'B', to ISO 9001 which provides packaging materials to your organisation. It is 4 pm and the audit is close to an end; you are having an internal meeting with the team to decide what will be presented to the auditee during the Closing meeting. The Closing meeting was scheduled at 5 pm.
You, as Audit Team Leader, audited top management. You explain to the audit team that you identified two nonconformities:
a. There is no documented information on Top Management Reviews, as required in clause 9.3 of ISO 9001:2015.
b. There is no evidence of Top Management Commitment as required in clause 5.1 of ISO 9001:2015. (e.g., not ensuring the availability of resources
to operate the QMS, not ensuring the establishment of objectives, no promotion of improvement, no promotion of the process approach).
All agreed to present these two nonconformities. They went to meet the Top Management of 'B' and noticed that the General Manager and three other managers (Production, Human Resources, and Sales) were present in the meeting room.
Considering the seriousness of the two nonconformities to Top Management, as audit team leader, from the following select the best option:
You are conducting an ISO 9001 audit of a Materials Recycling Facility. The organisation processes
waste plastics into raw materials for plastic bottle manufacturers. You reach the manual picking line
where operators are removing contaminant materials from incoming products, such as plastic bags,
plastic film and badly contaminated items that would compromise the recycling process. You
interview the line supervisor.
You: "Why are these plastic items being rejected at this stage?"
Auditee: "They do not meet our processing standards."
You: "What is the reason for that?"
Auditee: "These items are likely to damage the machinery down the line. They can also
compromise our quality standards. We need to protect our reputation for good quality output
materials."
You: "What happens to the rejected items?"
Auditee: "Some get melted down in another process later on, and some are disposed of as waste
products that cannot be recycled."
You: "What happens to the waste products?"
Auditee: "I'm not sure. I suppose they go to landfill."
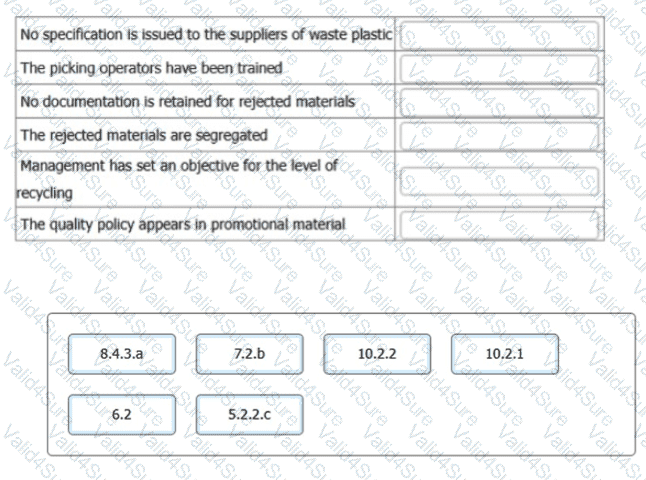
After further auditing, you have gathered additional evidence. Match the following
statements to the correct ISO 9001 standard clause shown.
To complete the table, click on the blank section you want to complete so that it is
highlighted in red, and then click on the applicable text from the options below.
Alternatively, drag and drop each option to the appropriate blank section.