CIC Exam Dumps - CBIC Certified Infection Control Exam
Searching for workable clues to ace the CBIC CIC Exam? You’re on the right place! ExamCert has realistic, trusted and authentic exam prep tools to help you achieve your desired credential. ExamCert’s CIC PDF Study Guide, Testing Engine and Exam Dumps follow a reliable exam preparation strategy, providing you the most relevant and updated study material that is crafted in an easy to learn format of questions and answers. ExamCert’s study tools aim at simplifying all complex and confusing concepts of the exam and introduce you to the real exam scenario and practice it with the help of its testing engine and real exam dumps
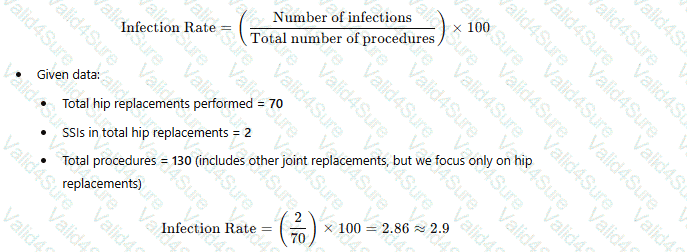
Operating room records indicate that 130 joint replacements have been performed. These include 70 total hip replacements, 55 total knee replacements, and 5 shoulder replacements. Two postoperative surgical site infections (SSIs) were identified in total hip replacements. What is the infection rate/100 procedures for total hip replacements?
An outbreak of carbapenem-resistant Klebsiella pneumoniae is linked to duodenoscopes. What is the infection preventionist’s PRIORITY intervention?
During the last week in June, an emergency department log reveals numerous cases of profuse watery diarrhea in individuals 74 years of age and older. During the same time period, four immunocompromised patients were admitted with possible Cryptosporidium. Which of the following actions should the infection preventionist take FIKST?
Following an outbreak of Hepatitis A, the water supply is sampled. A high count of which of the following isolates would indicate that the water was a potential source?
Immediate use steam sterilization is NOT recommended for implantable items requiring immediate use because
Which of the following BEST demonstrates the effectiveness of a program targeted at reducing central-line associated bloodstream infections (CLABSIs) in an intensive care unit (ICU)?
Which of the following activities will BEST prepare a newly hired infection preventionist to present information at the facility’s orientation program?